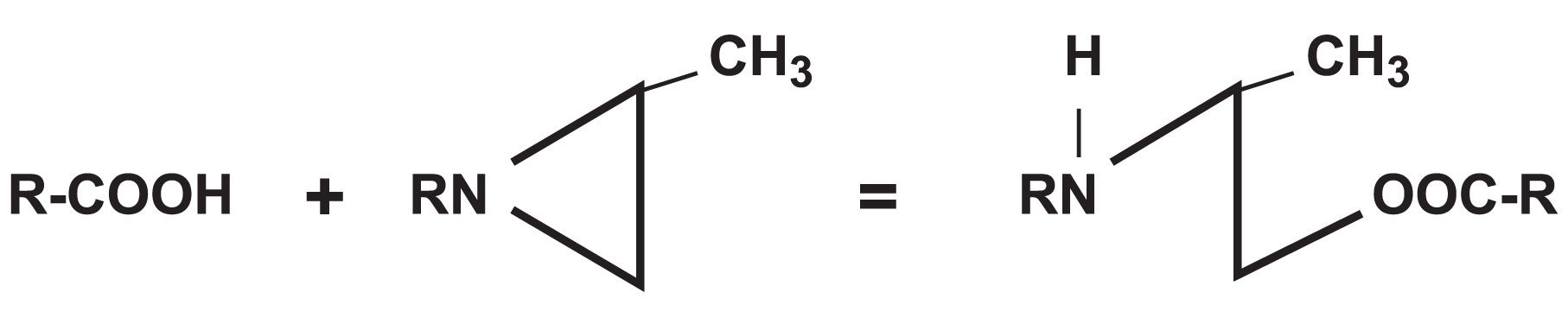
Polyfunctional Aziridine Reaction Mechanism
The reaction of polyfunctional aziridine cross-linkers are acid catalyzed. They are typically used to react with the carboxylic acid groups on acrylic adhesives. To cross-link, an active hydrogen must be available to open the aziridine ring. The reaction mechanism is shown below:
The active hydrogen on the carboxylic acid group on the acrylic or polyurethane resin reacts with the nitrogen of the polyfunctional aziridine, which opens the ring to cross-link the resin. Since polyfunctional aziridines are tri-functional and the acrylic emulsion of polyurethane dispersion can be multi-functional, a cross-link density or network is formed through this reaction mechanism.
The physical and chemical properties of acrylic emulsion or polyurethane dispersion based coatings, inks, and adhesives are increased due to the cross-link density.
The polyfunctional aziridine and carboxylic acid reaction will occur at ambient condition as the coating, ink, or adhesive dry. Adding heat in the drying process will increase the rate of reaction. As the system dries, the pH drops, which accelerates the reaction of the active hydrogen of the carboxylic acid with the polyfunctional aziridine.